封堵器 测试 - 心血管植入物
Questmed GmbH是心血管植入物和封堵器的测试实验室. 我们根据ISO22679、ASTM F3211、ISO25539-1、ISO25539-2和ISO25539-3测试心血管植入物(如 封堵器)的疲劳和耐久性以及其他机械载荷。对于轴向和流体动力载荷的疲劳和耐久性测试,我们使用我们认可的测试程序。
We are the first and only test lab with an accreditation for Occluder - Durability under axial and hydrodynamic load testing.
我们从2011年开始就有心脏闭合装置耐久性测试的经验,例如
- 卵圆孔未闭 封堵器 (PFO),
- 房间隔缺损 封堵器 (ASD),
- 动脉导管未闭 封堵器 (PDA),
- 室间隔缺损 封堵器 (VSD),
- 人工瓣周漏 封堵器 (PVL),
- 左心耳 封堵器 (LAA),
- 的心房流量調節器 (AFR IASD).
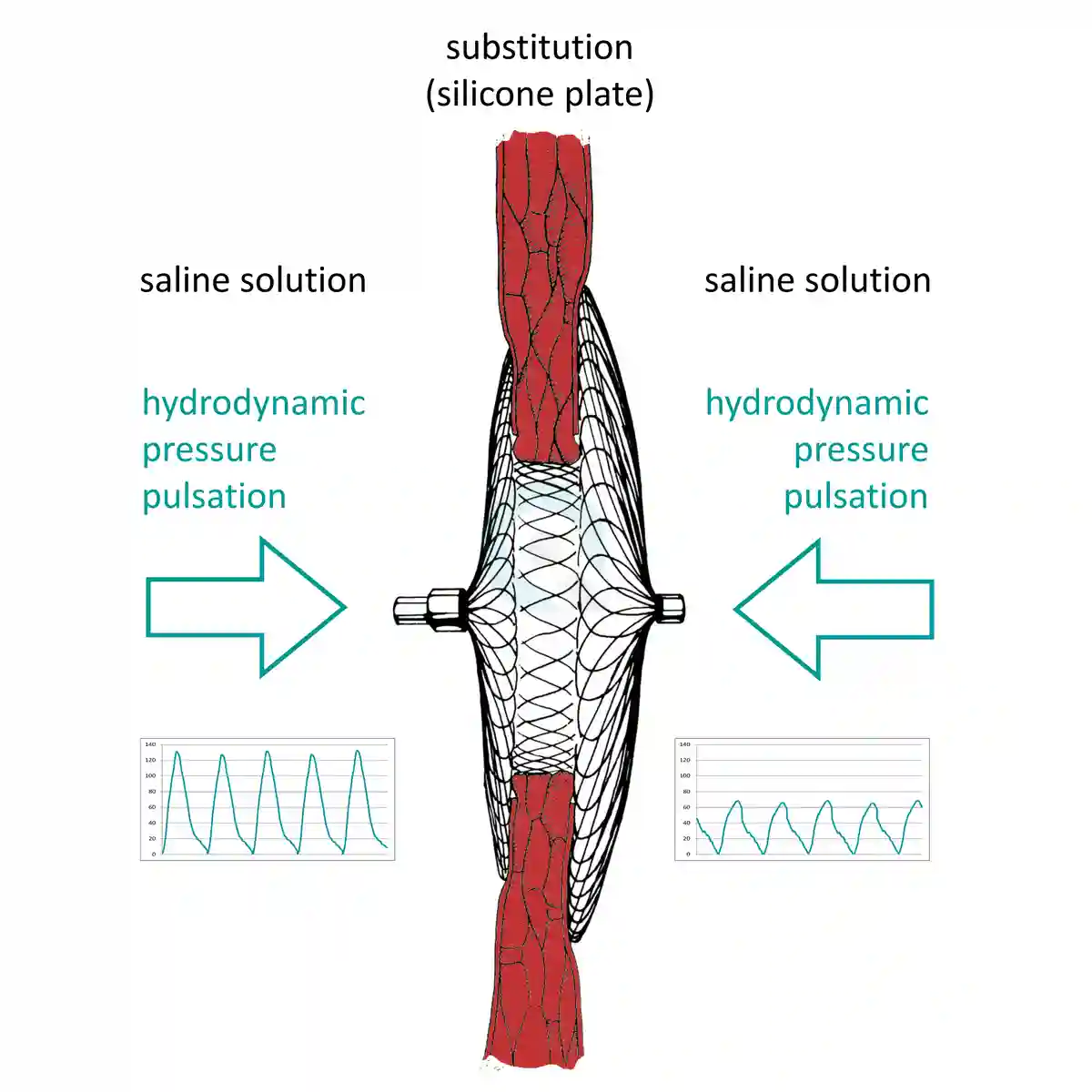
流体动力学压差脉动测试
The test should run according ISO 22679 Annex H.3 "Durability of the occluder implant".Fatigue and durability tests runs with 1.2 Hz up to 90 Hz at 37 °C tempered 0.9 % saline solution (ringer's solution).
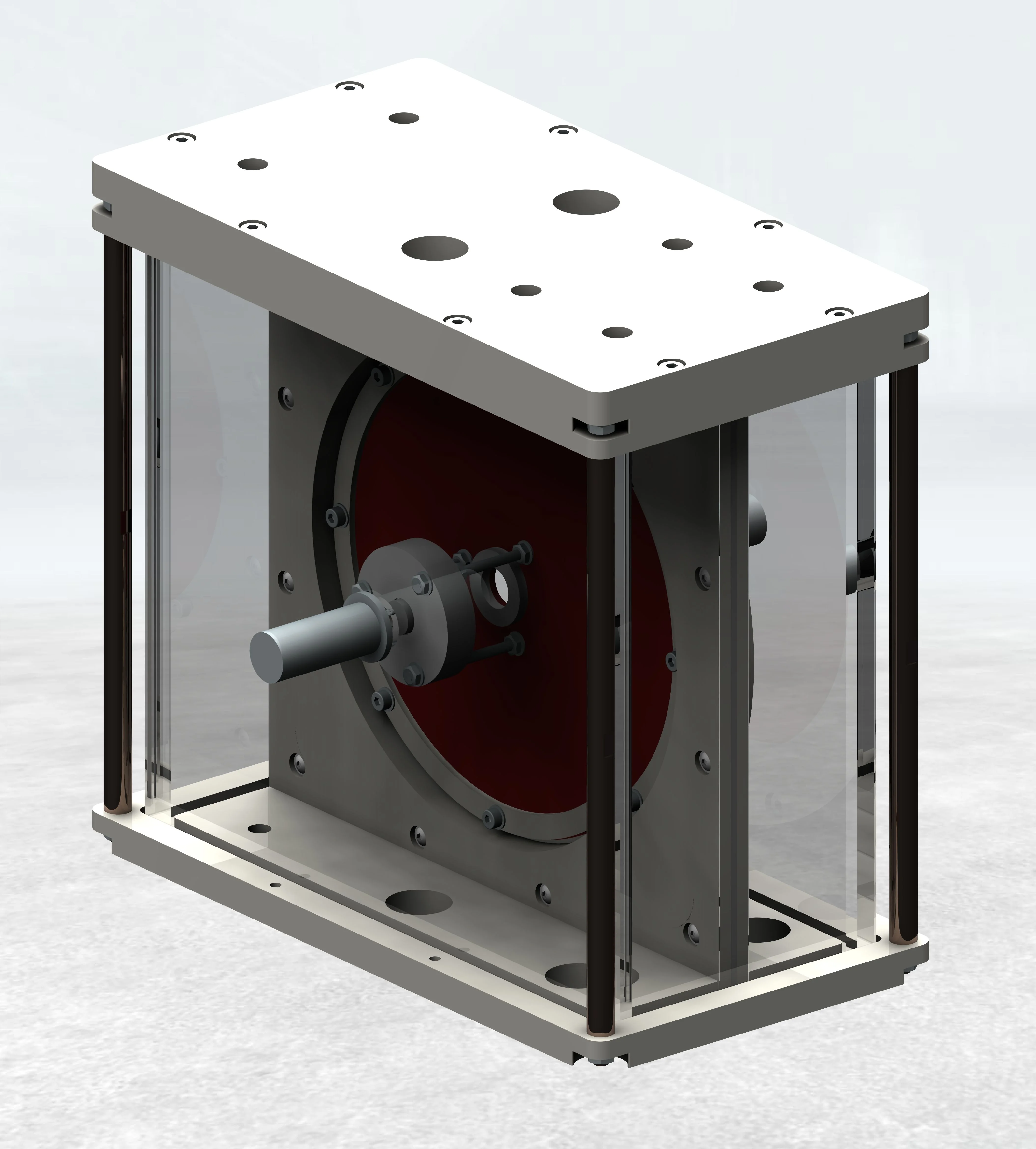
Our multidevice cardiac implant test systems for Occluder are available for 1 up to 30 Samples.
Occluder tempered at 37 °C blood heat is guaranteed by the fluid flow around the occluder by the unique desing of the testchamber.
High cycle fatigue and durability testing demonstrates the Occluder´s ability to withstand physiological stresses during long term implantation.
We perform pulsatile fatigue and durability testing for 10 million up to 600 million cycles.
Our test systems provides highspeed pressure tests with differential hydrodynamic pressures to the occluder with highspeed data recording to test the performance of Occluders using the Questmed OTV test system.
Pulsatile flows simulate the intended deployment location for Occluder and Closure Devices.
Steady flow will also be performed to better understand the fundamental operation of devices.
Tests include:
- Fatigue testing
- Durability testing
- Short-term and long-term pressurization
- Interim visual inspections without removing the test samples
- Stroboscope Visual Inspection
- Metal ion release analysis like nickel and titanium
用于疲劳和耐久性测试的压差脉动,封堵器
- 类型 - 压差脉动 (ISO 22679 附件 O)
- PFO - diff 14 mmHg
- ASD - diff 14 mmHg
- PDA - diff 110 mmHg
- VSD - diff 110 mmHg
- PVL - diff 119 mmHg
- LAA - diff 27 mmHg (持续性心房颤动)
- LAA - diff 18 mmHg (禁忌 心房颤动)
- AFR - diff 30 mmHg (interatrial shunt device IASD)
- AAA - diff 20 mmHg
轴向疲劳和耐久性测试
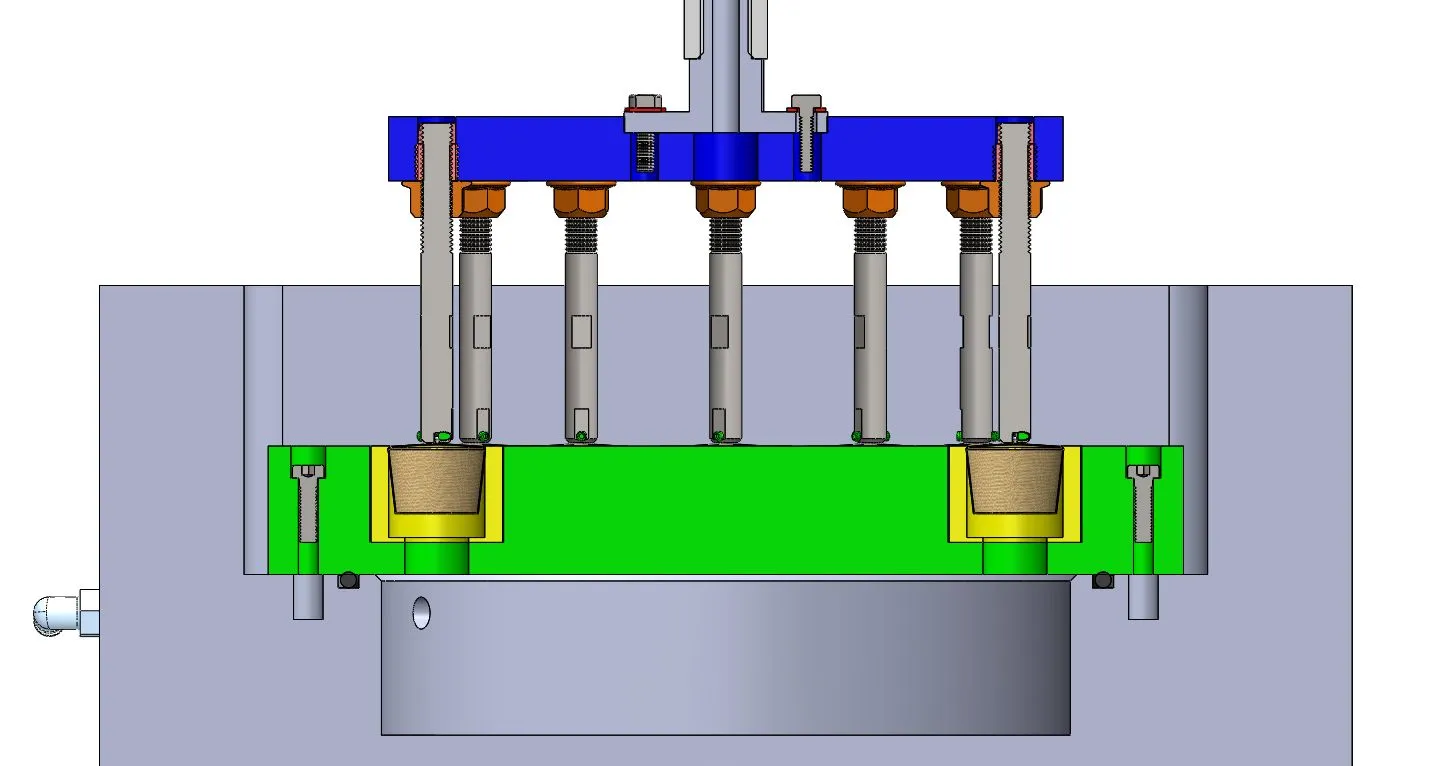
Axial tests runs with 10 Hz up to 90 Hz in 37 °C tempered 0.9 % saline ringer's solution.Our multidevice cardiac implant test system for Occluders is available for 12 Samples.
生物相容性 - 镍离子释放
We perform nickel ion release test according ASTM F3306 in accordance with Chapter III. C. 1. 生物相容性 - 镍离子释放 FDA Guidance Document 1545, Select Updates for Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems Guidance for Industry and Food and Drug Administration Staff Document issued on: August 18, 2015Questmed 封堵器 技能 / 專門技術
下载Questmed的演示文稿 封堵器 测试 技能 / 專門技術, presented at DIN Workinggroup Session 2021-06-18 - NA 027-05-06 AA "Herz- und Gefäßimplantate".下载 "Questmed 封堵器 技能 / 專門技術" 为PDF.